LIMS 2.0
Browser Based Application
Summary
Working alongside the product owner, stakeholders and users, I helped enhance the experience for one of the lab’s most important pieces of software. All the while, ensuring compliance and security of data could be verified.
Responsibilities
User Research
UI/UX Design
Project Management
Technical Writer
Verification and Validation
Background
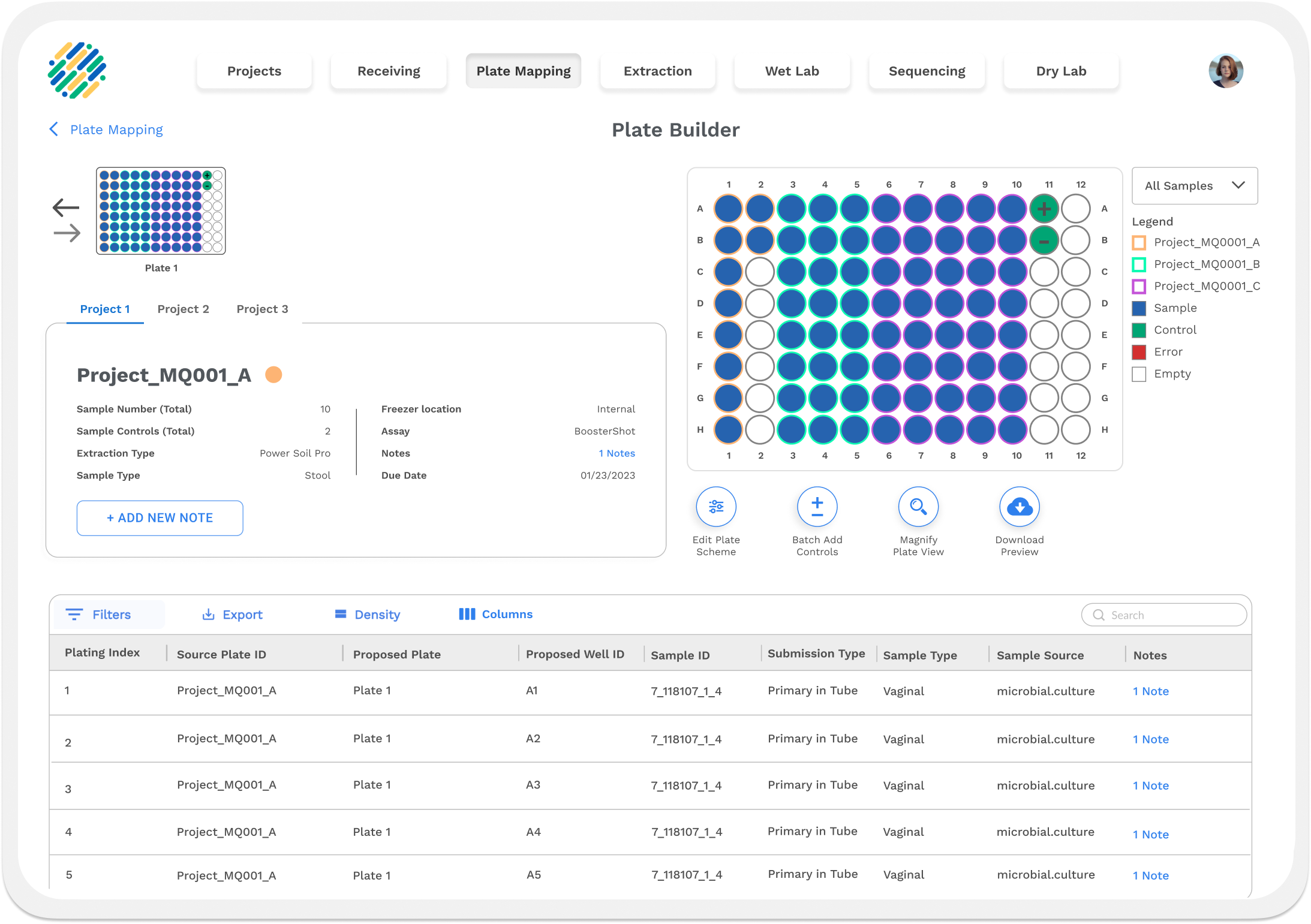
Within a laboratory setting it is crucial to have a way to track samples, manage data, and streamline workflows; this is where the Laboratory Information Management System comes into play. LIMS is a platform service with modular functionality that can be attached or removed depending on the labs needs.
Diversigen’s LIMS is a boutique creation, offering complete customization for the lab user’s needs. The director of operations at the lab is the product owner, prioritizing new features and work to be done during regular sprints. Stakeholders are typically subject matter experts in wet lab actives and help ensure that the LIMS follows lab processes. To ensure LIMS met the lab’s various compliance needs, verification and validation documentation is needed for larger feature sets and additional modulars.
Track, Manage, Streamline.
Track, Manage, Streamline.
Research, Rinse, and Repeat
As the sole UX/UI designer at Diversigen, I had many responsibilities. First and foremost, my role is to always advocate and understand the user’s needs. At Diversigen, I got the opportunity to work with both internal users and external users, or customers as we called them.
LIMS is a completely internal application with folks from many different roles accessing it so capturing separate persons was necessary to understand their unique perspective and needs. At Diversigen, the internal users included lab technicians, scientists, bioinformaticians, Quality assurance, lab managers and many more.
Each user comes with specific needs of LIMS. I had the distinct pleasure of shadowing many as they performed their daily work, watching closely to capture key moments and observing how they could be translated to digital solutions with LIMS.
KEY LIMS CONSIDERATIONS
-
Lab technicians have very limited bench space and almost always are wearing gloves when performing tasks. It is crucial to ensure that LIMS not only performs well on tablet , but looks good as well.
-
Traceability is paramount to the lab continuing work for high level customer. LIMS continuously needs to have sample level traceability to ensure that the lab meets compliance and regulatory needs.
-
LIMS routinely needs to call on data and showcase it in a meaningful way. Pagination and precise API usage helps LIMS stay nimble.
-
Labs are routinely audited to ensure they are keeping up with the compliance and regulatory needs of their customers. An Audit Tracker modular is included so that usage of LIMS can be logged and monitored at all times.
-
LIMS is designed not only to handle individual samples, but entire customer projects. This greatly helps the lab schedule and manage it’s work.
-
LIMS deals with a lot of types of data, and to ensure that the data captured meets quality assurance and security requirements, verification and validation documents are created for most features.
continuous integration & continuous deployment
continuous integration & continuous deployment
Fueling LIMS with agile scrum and CI/CD
Embedded within the software engineering team as the only UX designer, I consistently worked a few sprints ahead to ensure the team and LIMS could continuously move forward. In some cases, I acted as the scrum master and even lead stakeholders meetings for the LIMS product.
Using a continuous integration & continuous deployment process, myself and the software engineering team were able to release updates to LIMS weekly, fixing bugs, making improvements, and showcasing new features for the users consistently.
-
The LIMS data fetching strategy needed to be improved, in order to cut out unneeded complexity and make the software perform more reliably.
-
In order to meet FDA 21 CFR Part 11 compliance and regulatory needs, LIMS needed to include a required form of digital signature that would be invoked with any change made to LIMS data.
-
LIMS needed to include proper workflows for the new metatranscriptomics services, so that the lab technicians can properly document customer work for the new service offering.
-
LIMS needed to include a Dry Lab modular, so that bioinformatic and analysis work could be captured and audited within the LIIMS database.
-
LIMS needed the ability to process multiple projects with like assays for extraction, in order to best optimize plate constraints, as well as save time and money during the extraction steps within the lab.